Signaling the Immune System to Eat Tumor Cells
Neuroblastoma is a cancer of immature nerve cells, and most commonly affects infants and children. In general, it has one of the lowest survival rates for pediatric cancers, as the five-year survival rate is 50% for high-risk neuroblastoma patients. Furthermore, 60% of the high-risk group will relapse, and their five-year survival rate drops down to less than 5%. Current treatments include chemotherapy combined with anti-GD2 antibodies that target GD2, a molecule overexpressed (present at high levels) in neuroblastoma. Antibodies can target molecules and block them from binding to whatever they normally bind to, thus inhibit their normal effect. Although prognosis is improved by such treatment, the survival rate is still poor, and also introduces severe side effects. Thus, more effective and safer therapies are necessary.
Researchers at Stanford discovered that coupling anti-GD2 with anti-CD47 antibodies produces a synergistic effect that significantly eradicates neuroblastoma in in vitro human cell lines and in vivo mouse models. That is, the combination of both antibodies proved more effective than either antibody alone. They also discovered the same in osteosarcoma, a bone cancer known for high GD2 expression. CD47 is a checkpoint molecule overexpressed on tumor cells and inhibits specialized immune cells called macrophages from carrying out phagocytosis, or “eating up,” of tumor cells. It turns out that macrophages are regulated by a balance of “eat me” or “don’t eat me” signals on tumor cells, and CD47 is a “don’t eat me” signal, contributing to tumor persistence. Since combining anti-CD47 with anti-CD20 antibodies was effective in treating another type of cancer, the researchers decided to see if utilizing a similar combination strategy would be effective against neuroblastoma.
Researchers at Stanford discovered that coupling anti-GD2 with anti-CD47 antibodies produces a synergistic effect that significantly eradicates neuroblastoma in in vitro human cell lines and in vivo mouse models. That is, the combination of both antibodies proved more effective than either antibody alone. They also discovered the same in osteosarcoma, a bone cancer known for high GD2 expression. CD47 is a checkpoint molecule overexpressed on tumor cells and inhibits specialized immune cells called macrophages from carrying out phagocytosis, or “eating up,” of tumor cells. It turns out that macrophages are regulated by a balance of “eat me” or “don’t eat me” signals on tumor cells, and CD47 is a “don’t eat me” signal, contributing to tumor persistence. Since combining anti-CD47 with anti-CD20 antibodies was effective in treating another type of cancer, the researchers decided to see if utilizing a similar combination strategy would be effective against neuroblastoma.
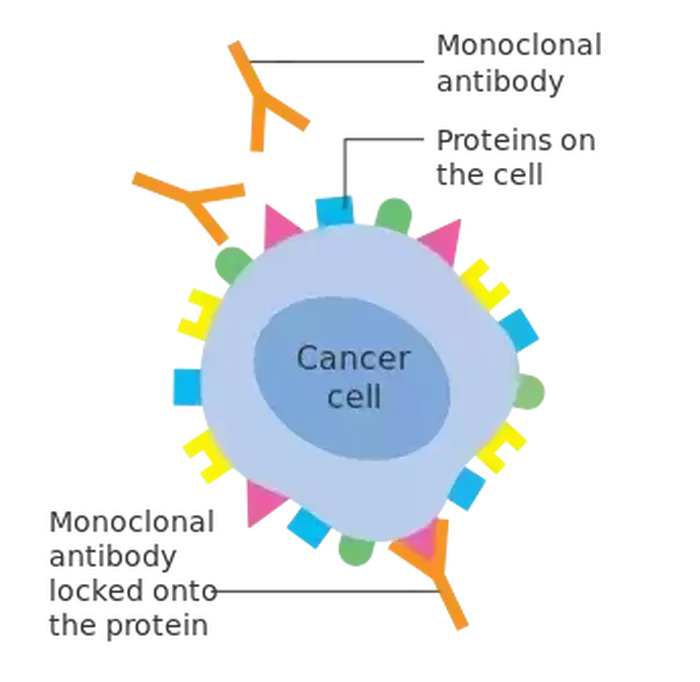
Image Source: "Monoclonal antibodies binding to cancer cell" by lswu is licensed under CC BY-SA 4.0
Indeed, with this combinatorial treatment in in vitro and in vivo models, the researchers noticed increased phagocytosis of tumor cells, as well as a significantly better survival rate of the mice. The researchers also observed a greater number of macrophages recruited to the tumor, with no toxic impact affecting the mice’s nervous system or other major organs. After all, GD2 is also expressed in normal tissue in humans and mice, and anti-GD2 only recognizes the GD2 molecule without differentiating between normal and cancerous tissue. Thus, potential toxic side effects must always be considered if this treatment is to be used for clinical applications.
Furthermore, the researchers investigated the potential mechanism behind this effect in greater detail. Interestingly, they discovered that GD2 normally binds to a molecule called Siglec-7, which can suppress macrophage activity—basically commanding the macrophages to “not eat” the tumor. Additionally, anti-GD2 antibody treatment results in higher surface levels of calreticulin, an “eat me” signal. They also verified previous findings which demonstrated that the GD2 binding to a ligand by itself, whether to its original target or a synthetic target (anti-GD2 antibody), induces tumor cell death. Collectively, these findings suggest that anti-GD2 treatment works by removing a “don’t eat me” signal, creating an “eat me” signal, and killing the tumor cells directly. Therefore, the additional anti-CD47 antibody treatment, which removes another “don’t eat me” signal, tips the balance towards favoring anti-tumor phagocytosis.
This treatment holds promise for greatly improving the survival rates of patients affected with GD2+ cancers like neuroblastoma and osteosarcoma, especially for those who are high-risk or relapsed. Currently, a clinical trial is underway to test the effectiveness and safety of this treatment. This treatment can be further refined by more investigation into the molecular mechanisms. Since the researchers found that the responsiveness of the treatment correlates with GD2 expression, which may vary between different patients, more research into how GD2 and CD47 expression impacts treatment effectiveness can prove to be beneficial for personalized treatments.
Furthermore, the researchers investigated the potential mechanism behind this effect in greater detail. Interestingly, they discovered that GD2 normally binds to a molecule called Siglec-7, which can suppress macrophage activity—basically commanding the macrophages to “not eat” the tumor. Additionally, anti-GD2 antibody treatment results in higher surface levels of calreticulin, an “eat me” signal. They also verified previous findings which demonstrated that the GD2 binding to a ligand by itself, whether to its original target or a synthetic target (anti-GD2 antibody), induces tumor cell death. Collectively, these findings suggest that anti-GD2 treatment works by removing a “don’t eat me” signal, creating an “eat me” signal, and killing the tumor cells directly. Therefore, the additional anti-CD47 antibody treatment, which removes another “don’t eat me” signal, tips the balance towards favoring anti-tumor phagocytosis.
This treatment holds promise for greatly improving the survival rates of patients affected with GD2+ cancers like neuroblastoma and osteosarcoma, especially for those who are high-risk or relapsed. Currently, a clinical trial is underway to test the effectiveness and safety of this treatment. This treatment can be further refined by more investigation into the molecular mechanisms. Since the researchers found that the responsiveness of the treatment correlates with GD2 expression, which may vary between different patients, more research into how GD2 and CD47 expression impacts treatment effectiveness can prove to be beneficial for personalized treatments.
Featured Image Source: ColiN00B
RELATED ARTICLES
|
Vertical Divider
|
Vertical Divider
|
Vertical Divider
|