New Hope to Combat Tumor Activity with Increased T-Cell Survival
T-cells play an important role in the immune system because they carry out a multitude of immune responses by recognizing virus-infected cells and attacking them directly. Once they are done with their job, T-cells self-destruct, but some are able to survive as memory T-cells. Memory T-cells induce long-term protection as they have “memory” of fighting against previously encountered pathogens. Metabolic activity is a factor in determining a T-cell’s fate.
Mitochondrial fatty acid oxidation is needed to generate memory T-cells. The mammalian target of rapamycin (mTOR) is the main regulator of cell metabolism and controls T-cell memory formation. Healthy metabolism and T-cell survival are needed to ensure the prevalence of anti-tumor responses. In a tumor microenvironment, T-cell dysfunction, stress, and cell death are frequent because of the scarce nutrients in the environment.
L-arginine levels affect T-cells in different ways. Increased intracellular L-arginine levels improves T-cell survival. It induces metabolic changes, which include decreasing glycolytic activity and increasing oxidative phosphorylation activity in activated T-cells. This activity may enhance memory T-cell formation and anti-tumor responses. However, L-arginine treated T-cells cause a delay in cell proliferation. T-cells can sense L-arginine depletion, which can occur in tumor microenvironments. A moderate reduction of L-arginine has a negative impact on T-cell survival without affecting proliferation. When L-arginine was completely depleted from the culture medium, T-cells no longer proliferated. Lack of L-arginine in T-cells can be sensed by GCN2, leading to an amino acid starvation response; it can also be sensed by SLC38A9, leading to the inhibition of T-cell growth and proliferation.
Mitochondrial fatty acid oxidation is needed to generate memory T-cells. The mammalian target of rapamycin (mTOR) is the main regulator of cell metabolism and controls T-cell memory formation. Healthy metabolism and T-cell survival are needed to ensure the prevalence of anti-tumor responses. In a tumor microenvironment, T-cell dysfunction, stress, and cell death are frequent because of the scarce nutrients in the environment.
L-arginine levels affect T-cells in different ways. Increased intracellular L-arginine levels improves T-cell survival. It induces metabolic changes, which include decreasing glycolytic activity and increasing oxidative phosphorylation activity in activated T-cells. This activity may enhance memory T-cell formation and anti-tumor responses. However, L-arginine treated T-cells cause a delay in cell proliferation. T-cells can sense L-arginine depletion, which can occur in tumor microenvironments. A moderate reduction of L-arginine has a negative impact on T-cell survival without affecting proliferation. When L-arginine was completely depleted from the culture medium, T-cells no longer proliferated. Lack of L-arginine in T-cells can be sensed by GCN2, leading to an amino acid starvation response; it can also be sensed by SLC38A9, leading to the inhibition of T-cell growth and proliferation.
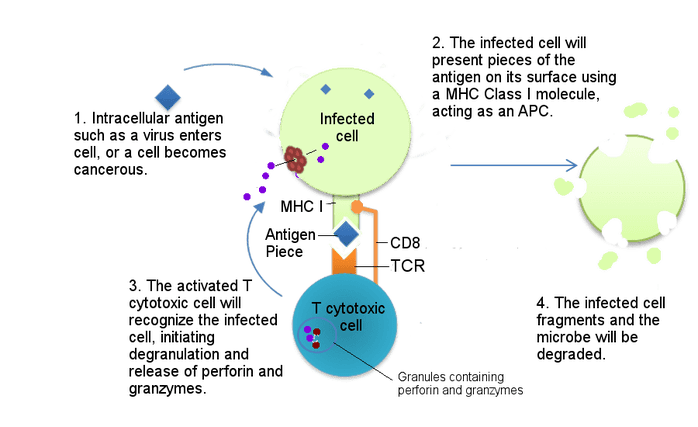
Image Source: "T cytotoxic interaction with infected cell" by Becky Boone is licensed under CC BY-SA 2.0
L-arginine also has other beneficial effects. For example, there was an increase in number and activity of natural killer cells in healthy volunteers who took L-arginine orally. L-arginine also improves mitochondrial function and reduces bronchial epithelial cell deaths after an allergic airway inflammation. It also has a beneficial effect for the treatment of patients with a mitochondrial disorder.
Three nuclear proteins (BAZ1B, PSIP1, and TSN) are crucial in T-cells for L-arginine to have an effect on T-cell survival. BAZ1B is a transcriptional regulator that binds to methylated histones. PSIP1 is a transcriptional co-activator that protects a cell from self-destruction. L-arginine causes structural changes in BAZ1B and PSIP1, which may affect DNA binding and lead to the initiation of T-cell survival. The last nuclear protein, TSN, is a small DNA and RNA binding protein that can influence cellular phenotypes. These three proteins had variable responses to L-arginine. This finding further confirms that L-arginine can be sensed by several independent proteins and can lead to increased T-cell survival.
In the future, this finding may offer the possibility of therapeutic applications. Genetic manipulations are no longer needed to cause a change in metabolite levels. The positive relationship between increased levels of L-arginine and T-cell survival may be exploited therapeutically. Lastly, it may lead to future studies of the complex relationship between metabolism and cellular functions.
Three nuclear proteins (BAZ1B, PSIP1, and TSN) are crucial in T-cells for L-arginine to have an effect on T-cell survival. BAZ1B is a transcriptional regulator that binds to methylated histones. PSIP1 is a transcriptional co-activator that protects a cell from self-destruction. L-arginine causes structural changes in BAZ1B and PSIP1, which may affect DNA binding and lead to the initiation of T-cell survival. The last nuclear protein, TSN, is a small DNA and RNA binding protein that can influence cellular phenotypes. These three proteins had variable responses to L-arginine. This finding further confirms that L-arginine can be sensed by several independent proteins and can lead to increased T-cell survival.
In the future, this finding may offer the possibility of therapeutic applications. Genetic manipulations are no longer needed to cause a change in metabolite levels. The positive relationship between increased levels of L-arginine and T-cell survival may be exploited therapeutically. Lastly, it may lead to future studies of the complex relationship between metabolism and cellular functions.
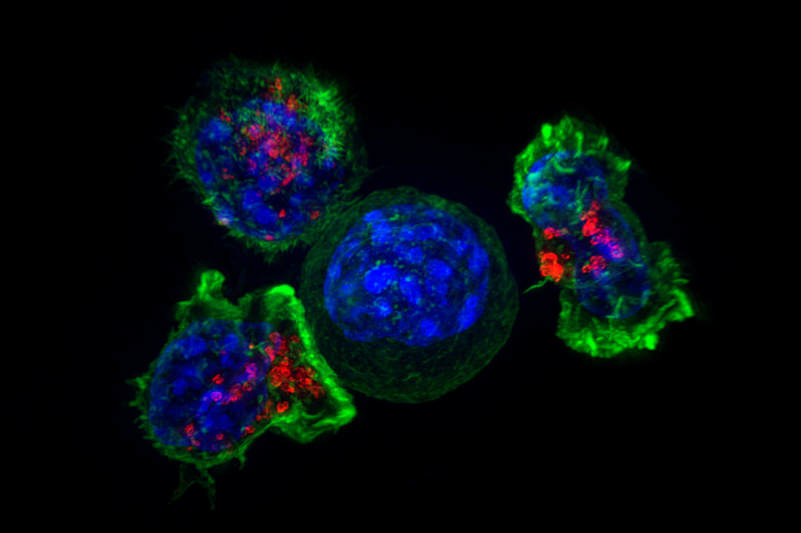
Featured Image Source: "Killer T cells surround a cancer cell" by NICHD is licensed under CC BY-NC-ND 2.0
RELATED ARTICLES
|
Vertical Divider
|
Vertical Divider
|
Vertical Divider
|