Intratumoral Microbiome and its Role in Cancer
The tumor microenvironment (TME)—or the environment around the tumor consisting of numerous components such as different kinds of cells, blood vessels, or extracellular matrix—plays a significant role in the development of cancer. Complex interactions between the tumor and the TME contribute to cancer progression. While for decades, the gut microbiome has been well-characterized in general and in connection to cancer, recent research has also illuminated the role of the intratumoral microbiome (the group of microorganisms residing in the tumor itself) in the TME of various cancer types. However, not much is known in detail about the identities of the intratumoral microbiota and their interacting cell types, nor whether the spatial distribution of this microbiome and its interactions influence the TME’s contributions to cancer.
This knowledge gap is filled by a study done by researchers at the Fred Hutchinson Cancer Center. They focused on patient tumor samples from 2 types of gastrointestinal tract-related cancers, oral squamous cell carcinoma (OSCC) and colorectal cancer (CRC). As a starting point of their analysis, they found that there was significant heterogeneity in the spatial distribution and identities of the intratumoral microbiota. That is, instead of all of the bacteria being randomly dispersed throughout the tumor, there was a diverse mix of different kinds of bacteria in varying proportions and sublocations.
Next, these researchers decided to look at whether this heterogeneity is linked to different functions in the TME. Sequencing technology was used to compare differences in amounts of protein involved in cancer development in areas without bacteria to areas with a lot of bacteria. Indeed, in such bacteria-enriched areas of the tumor, they found increased amounts of proteins connected to the inhibition of immune activity, which can contribute to cancer progression. Additionally, bacteria-enriched areas contained greater amounts of myeloid cells (another kind of white blood cell) and lesser amounts of T cells, which may indicate altered immune function
This knowledge gap is filled by a study done by researchers at the Fred Hutchinson Cancer Center. They focused on patient tumor samples from 2 types of gastrointestinal tract-related cancers, oral squamous cell carcinoma (OSCC) and colorectal cancer (CRC). As a starting point of their analysis, they found that there was significant heterogeneity in the spatial distribution and identities of the intratumoral microbiota. That is, instead of all of the bacteria being randomly dispersed throughout the tumor, there was a diverse mix of different kinds of bacteria in varying proportions and sublocations.
Next, these researchers decided to look at whether this heterogeneity is linked to different functions in the TME. Sequencing technology was used to compare differences in amounts of protein involved in cancer development in areas without bacteria to areas with a lot of bacteria. Indeed, in such bacteria-enriched areas of the tumor, they found increased amounts of proteins connected to the inhibition of immune activity, which can contribute to cancer progression. Additionally, bacteria-enriched areas contained greater amounts of myeloid cells (another kind of white blood cell) and lesser amounts of T cells, which may indicate altered immune function
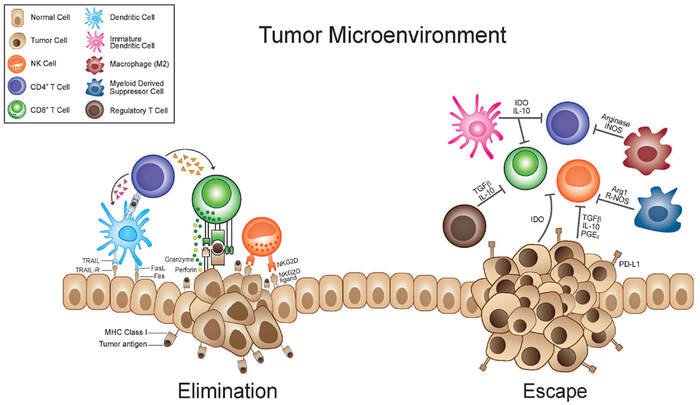
The tumor microenvironment (TME) involves a complicated interplay of dynamics between many different cell types and molecules. However, the tumor may evolve to escape elimination. Thus, all kinds of strategies like targeting the intratumoral microbiome should be considered for maximizing therapeutic possibilities and potential.
Then, within the TME, the researchers specifically examined interactions between bacteria and the cells that they adhere to or infect. In tumor samples from seven OSCC patients, they found a strong association between the bacterial species Fusobacterium and Treponema and with epithelial and macrophage cell clusters. Additionally, in these bacteria-positive epithelial cells, they discovered the increased activity of signaling pathways involved in cancer progression. As for the macrophages, their current technology was unable to tell if it was the bacteria that infected the macrophages or if it was the macrophages that phagocytized the bacteria, but they were able to find increased inflammatory signaling pathways in the bacteria-positive macrophages. These findings suggest how exactly the bacteria influence the function of these cells.
They also performed an in-vitro assay looking at how the intratumoral microbiome may directly affect host cell properties and functions. CRC tumor cells infected with a bacterial species recruited neutrophils (a type of white blood cell) and became more invasive, or acquired a greater ability to invade surrounding areas outside of their origin. Furthermore, these infected tumor cells were found to increase the activation of signaling pathways that promoted cancer development and decrease the activation of pathways that would deter cancer development. This serves as an example of how bacteria may directly promote cancer.
Overall, this study demonstrated a correlation between the heterogeneity of intratumoral microbiota and cancer. More research studies investigating their causal relationships, as well as in other types of cancers besides just these two gastrointestinal cancers, can further shed light on the cellular and molecular contributions of intratumoral microbiota to cancer. By obtaining a deeper understanding of this phenomenon, therapeutic and preventative remedies can be developed targeting elements of the intratumoral microbiota.
They also performed an in-vitro assay looking at how the intratumoral microbiome may directly affect host cell properties and functions. CRC tumor cells infected with a bacterial species recruited neutrophils (a type of white blood cell) and became more invasive, or acquired a greater ability to invade surrounding areas outside of their origin. Furthermore, these infected tumor cells were found to increase the activation of signaling pathways that promoted cancer development and decrease the activation of pathways that would deter cancer development. This serves as an example of how bacteria may directly promote cancer.
Overall, this study demonstrated a correlation between the heterogeneity of intratumoral microbiota and cancer. More research studies investigating their causal relationships, as well as in other types of cancers besides just these two gastrointestinal cancers, can further shed light on the cellular and molecular contributions of intratumoral microbiota to cancer. By obtaining a deeper understanding of this phenomenon, therapeutic and preventative remedies can be developed targeting elements of the intratumoral microbiota.
Featured Image Source: geralt
RELATED ARTICLES
|
Vertical Divider
|
Vertical Divider
|
Vertical Divider
|