The Potential of Nanotubes in Immunotherapy for Atherosclerosis
Atherosclerosis is the buildup of arterial plaque over an extended period of time, which can lead to clogged arteries. Atherosclerosis may result in a heart attack or stroke if the accumulated plaque blocks an artery that supplies blood and oxygen to the heart or brain respectively. In the United States, someone suffers a heart attack every 40 seconds, and cardiovascular disease is currently the leading cause of death worldwide. Therefore, there is a critical need to better understand the factors involved in atherosclerotic plaque buildup so that more effective therapies can be developed.
Growing evidence suggests that persistent inflammation triggers the formation of life-threatening atherosclerotic plaques. When cells in the plaque are dying they become inflamed, and once those cells die, their inflammation may spread to healthy cells. Overall, this leads to more cell death in a cycle of chronic inflammation. As such, the body relies on a highly important routine process called efferocytosis, in which cells of the immune system eat the dead or dying cells to clear away inflammation. However, the process of efferocytosis becomes impaired in arteries seriously affected by atherosclerotic disease.
Recently, a team of researchers at the Stanford University School of Medicine investigated a potential therapeutic strategy to decrease the persistent inflammation in atherosclerotic arteries. The researchers discovered that CD47-SIRPα signaling, a mechanism that promotes the onset and spreading of cancer, also impairs the process of efferocytosis in atherosclerotic patients. CD47-SIRPα signaling is increased in atherosclerosis, which prevents the immune cells from removing the dying and inflamed cells in the arterial plaque. In other words, increased CD47-SIRPα signaling contributes to the chronic inflammation characteristically found in dangerous plaques.
Growing evidence suggests that persistent inflammation triggers the formation of life-threatening atherosclerotic plaques. When cells in the plaque are dying they become inflamed, and once those cells die, their inflammation may spread to healthy cells. Overall, this leads to more cell death in a cycle of chronic inflammation. As such, the body relies on a highly important routine process called efferocytosis, in which cells of the immune system eat the dead or dying cells to clear away inflammation. However, the process of efferocytosis becomes impaired in arteries seriously affected by atherosclerotic disease.
Recently, a team of researchers at the Stanford University School of Medicine investigated a potential therapeutic strategy to decrease the persistent inflammation in atherosclerotic arteries. The researchers discovered that CD47-SIRPα signaling, a mechanism that promotes the onset and spreading of cancer, also impairs the process of efferocytosis in atherosclerotic patients. CD47-SIRPα signaling is increased in atherosclerosis, which prevents the immune cells from removing the dying and inflamed cells in the arterial plaque. In other words, increased CD47-SIRPα signaling contributes to the chronic inflammation characteristically found in dangerous plaques.
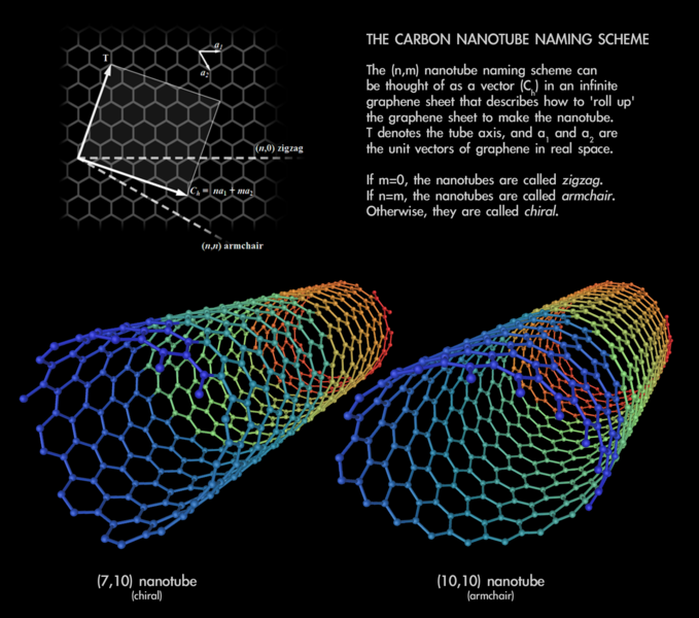
To safely and specifically restore efferocytosis that is impaired in atherosclerotic plaques, the researchers engineered SWNT-SHP1i nanotubes. Single-walled carbon nanotubes (SWNTs) are microscopic cylindrical molecules formed by rolling up a sheet of single-layer carbon atoms called graphene. These SWNTs were loaded with a chemical that would act on a molecule called SHP-1 to decrease the CD47-SIRPα signaling found in atherosclerotic plaques. The researchers believed that decreasing CD47-SIRPα signaling would restore the process of efferocytosis. This, in turn, would reduce the persistent inflammation associated with dangerous atherosclerotic plaques.
In order to assess if the SWNT-SHP1i system could have a therapeutic effect on atherosclerosis by reducing inflammation, researchers performed experiments in two different mouse models. The first type of mice had atherosclerotic plaque accompanied by inflammation in their arteries. The second type of mice had chronic inflammation not associated specifically with atherosclerosis. In both of these types of mice, efferocytosis was initially impaired because their immune cells were unable to clear away inflamed, dying cells. After weekly injections of SWNT-SHP1i nanotubes, a significant reduction in inflammation was observed in both mouse models. These experimental results suggest that SWNT-SHP1i nanotubes are able to decrease CD47-SIRPα signaling, which then restores efferocytosis so that the immune cells of the mice can remove the dying cells and inflammation in atherosclerotic plaques.
To conclude, injections of SWNT-SHP1i nanotubes have the potential to target and clear away inflammation in atherosclerotic plaques without compromising safety in mouse models. Decreasing this chronic inflammation may also decrease the buildup of dangerous arterial plaques in clogged arteries. If the experimental results obtained in mouse models can eventually be replicated in humans, this research presents a promising alternative strategy in the treatment and prevention of atherosclerotic cardiovascular disease.
In order to assess if the SWNT-SHP1i system could have a therapeutic effect on atherosclerosis by reducing inflammation, researchers performed experiments in two different mouse models. The first type of mice had atherosclerotic plaque accompanied by inflammation in their arteries. The second type of mice had chronic inflammation not associated specifically with atherosclerosis. In both of these types of mice, efferocytosis was initially impaired because their immune cells were unable to clear away inflamed, dying cells. After weekly injections of SWNT-SHP1i nanotubes, a significant reduction in inflammation was observed in both mouse models. These experimental results suggest that SWNT-SHP1i nanotubes are able to decrease CD47-SIRPα signaling, which then restores efferocytosis so that the immune cells of the mice can remove the dying cells and inflammation in atherosclerotic plaques.
To conclude, injections of SWNT-SHP1i nanotubes have the potential to target and clear away inflammation in atherosclerotic plaques without compromising safety in mouse models. Decreasing this chronic inflammation may also decrease the buildup of dangerous arterial plaques in clogged arteries. If the experimental results obtained in mouse models can eventually be replicated in humans, this research presents a promising alternative strategy in the treatment and prevention of atherosclerotic cardiovascular disease.
RELATED ARTICLES
|
Vertical Divider
|
Vertical Divider
|
Vertical Divider
|