The First Step Towards Prescribing Cannabis for Seizures
On November 9, 2016, over half of California voters opted to legalize recreational cannabis sales and use in their state. Beginning in January, 2018, adults aged 21 years or older can purchase cannabis from licensed stores and use it recreationally. This proposition was designed to increase state revenue through taxes, provide a safe environment for an increasing marijuana usage, and decrease black market activity. However, other proponents of legal cannabis, including some medical doctors, pharmaceutical companies, and epilepsy patients have found a use for cannabis beyond recreational use.
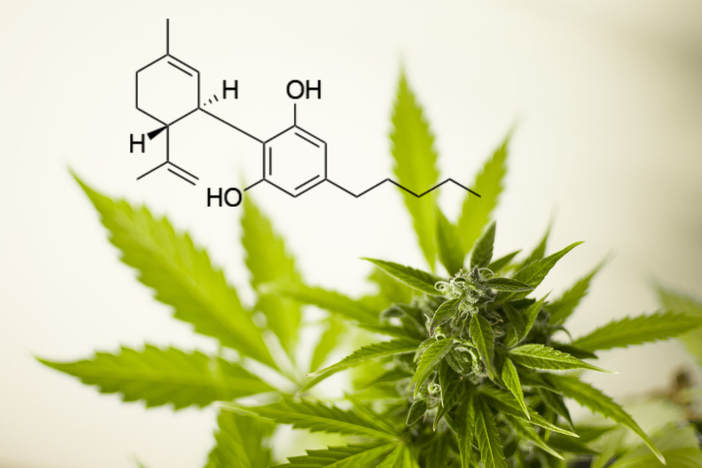
Decades of research on epilepsy patients have shown that concentrated cannabidiol (CBD) oil extracted from cannabis plants can control and diminish seizures. CBD is a non-psychoactive component of the cannabis plant, meaning it does not produce a “high.” Researchers are not entirely sure how CBD oil works to reduce seizures, but it has been found to be especially effective in controlling seizures in two rare, but hard to treat, forms of epilepsy: Lennox-Gastaut syndrome (LGS) and Dravet syndrome.
On April 18 of this year, the United States Food and Drug Administration (FDA) unanimously voted to take steps in approving Epidiolex®, a pharmaceutical drug developed by GW Pharmaceuticals to treat Lennox-Gastaut syndrome (LGS) and Dravet syndrome. The medication is derived from cannabis plants and high in CBD. This medication has undergone years of testing, and the results have shown that it shows more benefit than harm in patients.
Decades of research on epilepsy patients have shown that concentrated cannabidiol (CBD) oil extracted from cannabis plants can control and diminish seizures. CBD is a non-psychoactive component of the cannabis plant, meaning it does not produce a “high.” Researchers are not entirely sure how CBD oil works to reduce seizures, but it has been found to be especially effective in controlling seizures in two rare, but hard to treat, forms of epilepsy: Lennox-Gastaut syndrome (LGS) and Dravet syndrome.
On April 18 of this year, the United States Food and Drug Administration (FDA) unanimously voted to take steps in approving Epidiolex®, a pharmaceutical drug developed by GW Pharmaceuticals to treat Lennox-Gastaut syndrome (LGS) and Dravet syndrome. The medication is derived from cannabis plants and high in CBD. This medication has undergone years of testing, and the results have shown that it shows more benefit than harm in patients.
If approved fully by the FDA to treat these rare forms of epilepsy, Epidiolex® will be the first cannabis-derived medication approved by the FDA. This is a huge milestone for establishing marijuana’s medicinal value. However it creates a paradox in how cannabis is legally treated. The United States Drug Enforcement Agency (DEA) has declared that marijuana (and therefore anything derived from it) is a Schedule I drug. According to the DEA, this means that it has no medicinal purpose and can easily be abused. This places cannabis in the same category as heroin and LSD. Further complicating the issue, if the FDA approves Epidiolex®, then the DEA must reschedule the pharmaceutical drug, but not marijuana, to become legal according to the Improving Regulatory Transparency for New Medical Therapies Act.
This year, cannabis has seen a shift from being an illicit recreational drug to a potential new therapy. Epidiolex® is the first cannabis-derived medication to show promising results yet still technically be considered illegal. The role that controlled substances have on public health will always rely on the balance between safe use and legality. For example, opioids can both be prescribed by doctors to treat pain and also send someone to the emergency room for an overdose. Careful research can lead to efficient public health outreach and allow medications to function as therapies and not as substances for abuse.
This year, cannabis has seen a shift from being an illicit recreational drug to a potential new therapy. Epidiolex® is the first cannabis-derived medication to show promising results yet still technically be considered illegal. The role that controlled substances have on public health will always rely on the balance between safe use and legality. For example, opioids can both be prescribed by doctors to treat pain and also send someone to the emergency room for an overdose. Careful research can lead to efficient public health outreach and allow medications to function as therapies and not as substances for abuse.
Featured Image Source: coltsfan and 7raysmarketing
RELATED ARTICLES
|
Vertical Divider
|
Vertical Divider
|
Vertical Divider
|