Stem Cells. The Cure or Not the Cure
The topic of stem cells has received substantial attention in recent news articles, TV programs, and radio shows. They have been deemed a cure for anything from spinal cord injuries to Alzheimer’s Disease. To discern the truth from falsehood, it is crucial to understand what stem cells are and what the health community really knows about them.
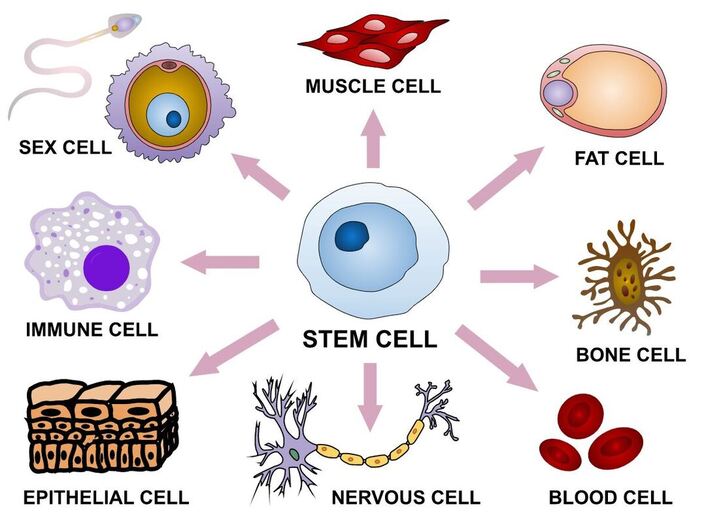
A cell is a basic functional unit of an organism. Stem cells are cells that have a unique ability to become any type of cell in the body. They can develop into muscles, bones, hair, blood, and all other organs. Stem cells can also divide and make numerous copies of themselves with virtually no limit. These remarkable properties of stem cells allow them to serve as a repair system for several tissues in the body and replace dead or diseased cells. Scientists are particularly excited about stem cells because, with the use of these cells, researchers can model and observe the development of various illnesses. In addition, stem cells can potentially be used to develop treatments for diseases that are currently not curable.
A cell is a basic functional unit of an organism. Stem cells are cells that have a unique ability to become any type of cell in the body. They can develop into muscles, bones, hair, blood, and all other organs. Stem cells can also divide and make numerous copies of themselves with virtually no limit. These remarkable properties of stem cells allow them to serve as a repair system for several tissues in the body and replace dead or diseased cells. Scientists are particularly excited about stem cells because, with the use of these cells, researchers can model and observe the development of various illnesses. In addition, stem cells can potentially be used to develop treatments for diseases that are currently not curable.
Numerous stem cell treatments are currently being developed for various diseases and disorders; however, the process of treatment development is very lengthy and demanding. Scientists must follow rigorous protocols implemented by the U.S. Food and Drug Administration (FDA) in order to ensure that the drugs are effective and safe to be used.
The first step along the Drug Discovery Pipeline is the Preclinical Studies. These studies are conducted on animals, and the researchers are required to gather a vast amount of information to justify that the potential treatment would be beneficial and safe to be administered in humans. Once enough data is collected, the investigators must submit their proposal and data to the FDA to receive permission to continue with the trials in human subjects. If the FDA finds the study’s evidence to be promising, compelling, and compliant with the established regulations, the organization will allow the study to proceed with the clinical trials that are designated to test the efficacy and safety of the proposed treatment in humans.
Although the process of clinical trials is highly regulated, controversies about stem cell research still exist. The FDA recently released a warning to notify the general public of the dangers posed by unproven and unapproved stem cell treatments. The FDA acknowledges the possibility that stem cells could help treat many diseases in the future, but as of now, the only stem-cell-based therapies approved by the FDA are used to treat disorders of the hematopoietic system (the organ system involved in the production of blood). If one is considering receiving a stem cell treatment or enrolling in a clinical trial, one should find out if the treatment is FDA approved or if the study has an approved Investigational New Drug Application (IND) — an application that must be authorized by the FDA in order for any new drugs or treatments to be administered to humans. All of the active and inactive clinical trials can be found on the following website: https://clinicaltrials.gov/; however, not all of the trials are approved by the FDA. The only clinical trials that are approved by the FDA are those that have an IND number, which can be obtained by contacting the designated clinical trial directly via phone or e-mail (please note that IND numbers are not listed on the clinicaltrials.gov website). The FDA continues to observe ongoing research and ensure product safety. Nevertheless, individuals seeking stem cell treatment should be cautious and gather all the evidence prior to proceeding with the intervention.
The first step along the Drug Discovery Pipeline is the Preclinical Studies. These studies are conducted on animals, and the researchers are required to gather a vast amount of information to justify that the potential treatment would be beneficial and safe to be administered in humans. Once enough data is collected, the investigators must submit their proposal and data to the FDA to receive permission to continue with the trials in human subjects. If the FDA finds the study’s evidence to be promising, compelling, and compliant with the established regulations, the organization will allow the study to proceed with the clinical trials that are designated to test the efficacy and safety of the proposed treatment in humans.
Although the process of clinical trials is highly regulated, controversies about stem cell research still exist. The FDA recently released a warning to notify the general public of the dangers posed by unproven and unapproved stem cell treatments. The FDA acknowledges the possibility that stem cells could help treat many diseases in the future, but as of now, the only stem-cell-based therapies approved by the FDA are used to treat disorders of the hematopoietic system (the organ system involved in the production of blood). If one is considering receiving a stem cell treatment or enrolling in a clinical trial, one should find out if the treatment is FDA approved or if the study has an approved Investigational New Drug Application (IND) — an application that must be authorized by the FDA in order for any new drugs or treatments to be administered to humans. All of the active and inactive clinical trials can be found on the following website: https://clinicaltrials.gov/; however, not all of the trials are approved by the FDA. The only clinical trials that are approved by the FDA are those that have an IND number, which can be obtained by contacting the designated clinical trial directly via phone or e-mail (please note that IND numbers are not listed on the clinicaltrials.gov website). The FDA continues to observe ongoing research and ensure product safety. Nevertheless, individuals seeking stem cell treatment should be cautious and gather all the evidence prior to proceeding with the intervention.
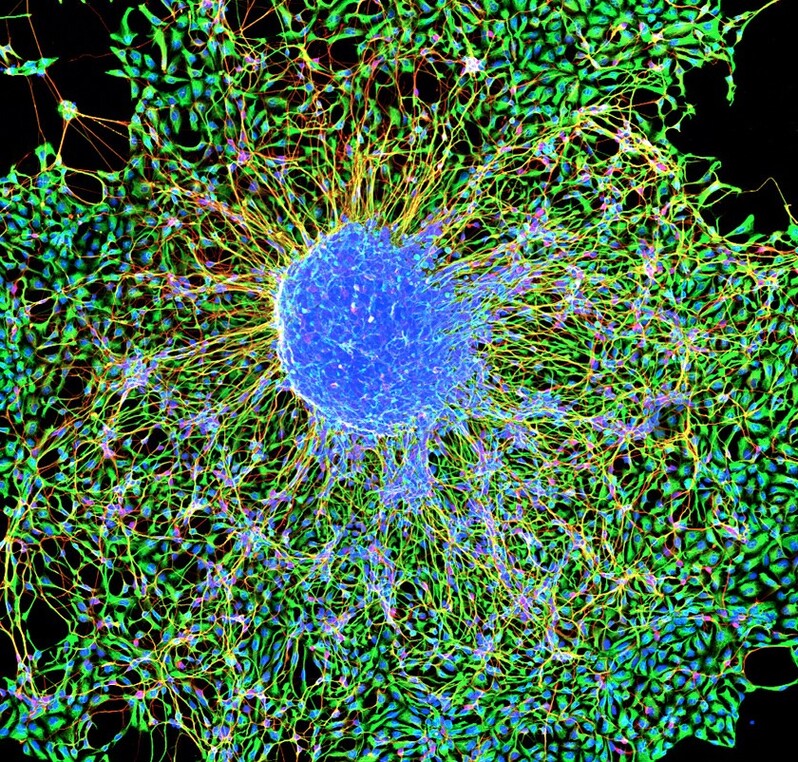
Featured Image Source: "Marmoset embryonic stem cells forming neurons" by NIH Image Gallery is licensed by CC BY-NC 2.0
RELATED ARTICLES
|
Vertical Divider
|
Vertical Divider
|
Vertical Divider
|