Rising Editing Tool for Genomic Disorders
The human body is built from millions of types of cells: each kind of cell is designed to carry out a specific function. Residing within the nucleus of many of these cells are genomic structures known as chromosomes. These chromosomal structures consist of packed deoxyribonucleic acids, commonly known as DNA. Genes, composed of certain segments of DNA, contain the information necessary to regulate protein synthesis, determining when and which proteins are created. Proteins are critical to human life since they determine the structure and function of cells through specialization. As the unit regulating protein synthesis, genes are crucial in ensuring that cells perform their tasks correctly.
According to the World Health Organization, there are approximately over 10,000 monogenic human disorders, diseases which result from mutations in a single gene sequence causing the modified cells to malfunction. These monogenic disorders are categorized into three groups: dominant (mutation of at least one gene), recessive (mutation of both copies of the gene) and X-linked (mutations on the X sex chromosome). Examples of monogenic diseases include sickle cell anemia, cystic fibrosis, Huntington’s disease, and Tay-Sachs disease.
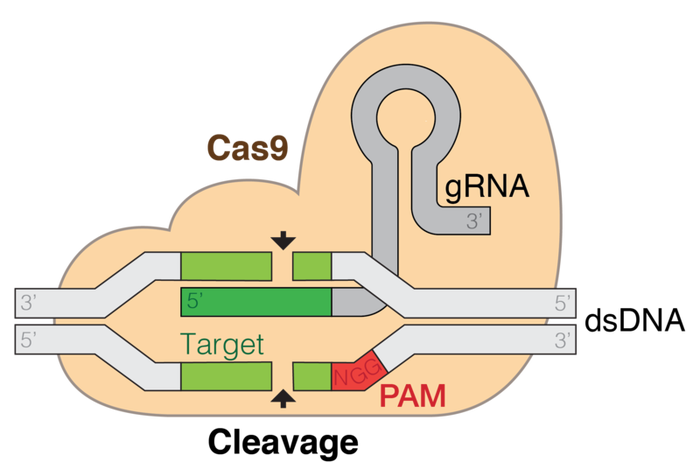
Recently, scientists have developed a genome editing technique known as CRISPR-Cas9, which allows the manipulation of DNA coding sequences. This process involves the Cas9 enzyme cutting DNA strands at specific locations in the genome sequence that are marked by the guide ribonucleic acid (gRNA). Cas9 creates a “nick” in the DNA that enables the addition or removal of nucleotides, units which make up DNA, thereby changing the gene sequence. Most researchers utilize this technique to introduce mutations and create gene knockouts.
According to the World Health Organization, there are approximately over 10,000 monogenic human disorders, diseases which result from mutations in a single gene sequence causing the modified cells to malfunction. These monogenic disorders are categorized into three groups: dominant (mutation of at least one gene), recessive (mutation of both copies of the gene) and X-linked (mutations on the X sex chromosome). Examples of monogenic diseases include sickle cell anemia, cystic fibrosis, Huntington’s disease, and Tay-Sachs disease.
Recently, scientists have developed a genome editing technique known as CRISPR-Cas9, which allows the manipulation of DNA coding sequences. This process involves the Cas9 enzyme cutting DNA strands at specific locations in the genome sequence that are marked by the guide ribonucleic acid (gRNA). Cas9 creates a “nick” in the DNA that enables the addition or removal of nucleotides, units which make up DNA, thereby changing the gene sequence. Most researchers utilize this technique to introduce mutations and create gene knockouts.
A recent study took a different approach by using this genome-editing tool to target and correct mutations in germline cells, the stage of cell development following egg cell fertilization. The researchers specifically worked to correct the heterozygous Myosin Binding Protein C (MYBPC3) mutation in embryos. Activated mainly in heart muscle cells, a normal MYBPC3 gene synthesizes proteins associated with the sarcomere, the basic unit of a myofibril (a structural unit within a muscle cell). Sarcomeres consist of thick and thin filaments which move relative to each other upon contraction and relaxation of the muscle. MYBPC3 specifically binds to thick filaments ensuring that these structures do not degrade prematurely. This protein also regulates cardiac muscle contraction and relaxation. MYBPC mutations result in myocardial diseases such as hypertrophic cardiomyopathy, which involves heart muscle stiffness, and myofibrillar disarray.
Using CRISPR-Cas9, a system with high-targeting accuracy thanks to gRNAs, the scientists aimed to introduce specific nicks called double-strand breaks (DSBs) in the DNA sequence within mutated regions of the MYBPC3 gene. This way, the genome can self-repair with the correct sequence when the DNA automatically repairs itself. The researchers aimed to improve the efficiency, accuracy, and safety of this technique by examining the DNA damage repair mechanisms in gametes, different cell cycle stages, and different embryonic stages. One of the pressing problems of a developing fertilized egg is mosaicism, a consequence in which groups of cells in an individual have different gene makeups. From their findings, they demonstrated that the use of CRISPR-Cas9 system at specific cell division stages resolves mosaicism, suggesting that gene editing efficiencies are highly affected by the cell cycle phases.
Researchers continue to explore and improve applications for CRISPR-Cas9. Although it has yet to be used as a common practice in clinical settings, CRISPR-Cas9 has been shown to be a promising tool in targeting genetic disorders.
Using CRISPR-Cas9, a system with high-targeting accuracy thanks to gRNAs, the scientists aimed to introduce specific nicks called double-strand breaks (DSBs) in the DNA sequence within mutated regions of the MYBPC3 gene. This way, the genome can self-repair with the correct sequence when the DNA automatically repairs itself. The researchers aimed to improve the efficiency, accuracy, and safety of this technique by examining the DNA damage repair mechanisms in gametes, different cell cycle stages, and different embryonic stages. One of the pressing problems of a developing fertilized egg is mosaicism, a consequence in which groups of cells in an individual have different gene makeups. From their findings, they demonstrated that the use of CRISPR-Cas9 system at specific cell division stages resolves mosaicism, suggesting that gene editing efficiencies are highly affected by the cell cycle phases.
Researchers continue to explore and improve applications for CRISPR-Cas9. Although it has yet to be used as a common practice in clinical settings, CRISPR-Cas9 has been shown to be a promising tool in targeting genetic disorders.
Featured Image Source: qimono
RELATED ARTICLES
|
Vertical Divider
|
Vertical Divider
|
Vertical Divider
|