Reversing the Age of Optic Neurons
Aging is an unrelenting and inevitable foe for everyone. The aging body is typically characterized by decreased strength, mobility, and immunity caused by the wear and tear of everyday life breaking down the bodies’ cellular and molecular functions. Scientists believe that the reason for these symptoms of old age lies in the realm of epigenetics, which is the study of how one’s behavior and environment can affect gene functions. One mechanism of epigenetic control is the carefully-controlled pattern of methylation, or “turning off,” of certain genes and regions of the DNA, which is essential for cell differentiation, the process by which cells mature and become fully functional. Over time, random points of methylation begin to accumulate, and what was once a perfectly synchronized factory of a cell becomes a malfunctioning hazard.
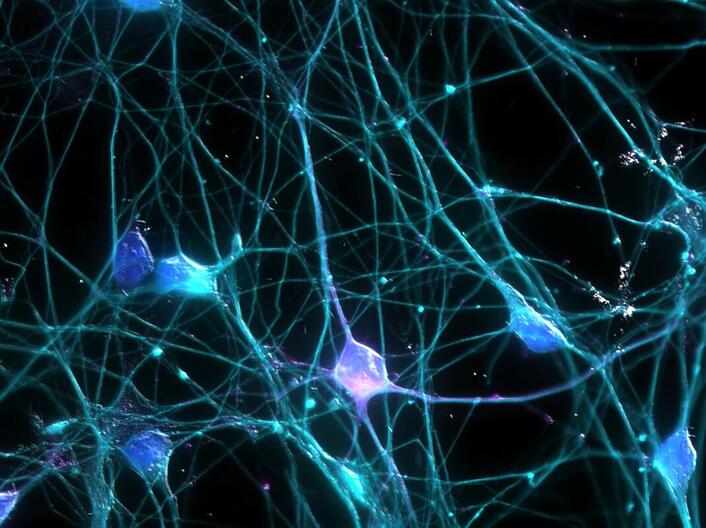
Recently, a team from Harvard Medical School returned the epigenome of aged or damaged neurons back to their youthful methylation pattern, thereby improving their functionality and regenerative capability. Previous studies have identified four proteins, known as the Yamanaka factors, that have the combined ability to completely erase methylation: OCT4, SOX2, OSK, and MYC. The researchers hypothesized that increased expression of three of the four proteins (OCT4, SOX2, and OSK) could reverse abnormal methylation while maintaining cell differentiation. In mice models, the increased expression of OSK safely returned cells to a youthful state. The team proceeded to express OSK in retinal ganglion cells (RGCs) that make up the eye’s optic nerve. Mice with treated RGCs showed signs of regeneration after injury, an ability held only by embryonic neurons. Thus, this demonstrates that OSK therapy can rewind the epigenetic clock of neurons in the optic nerve.
Recently, a team from Harvard Medical School returned the epigenome of aged or damaged neurons back to their youthful methylation pattern, thereby improving their functionality and regenerative capability. Previous studies have identified four proteins, known as the Yamanaka factors, that have the combined ability to completely erase methylation: OCT4, SOX2, OSK, and MYC. The researchers hypothesized that increased expression of three of the four proteins (OCT4, SOX2, and OSK) could reverse abnormal methylation while maintaining cell differentiation. In mice models, the increased expression of OSK safely returned cells to a youthful state. The team proceeded to express OSK in retinal ganglion cells (RGCs) that make up the eye’s optic nerve. Mice with treated RGCs showed signs of regeneration after injury, an ability held only by embryonic neurons. Thus, this demonstrates that OSK therapy can rewind the epigenetic clock of neurons in the optic nerve.
While healthy neurons expressing the OSK protein exhibit regenerative ability following injury, OSK’s potential to treat injury remained unclear. Damaged cells often show accelerated methylation similar to what occurs in rapid aging, so reversing methylation after injury could be the key to recovery. To test this, the researchers induced mice with damaged RGCs to produce more OSK. They found that increased OSK production greatly increased RGC self-repair compared to injured nerves without OSK. This conclusion carried over to human neurons, where the cells were able to recover from chemotherapy-induced injury. None of the mice RGCs or human neurons displayed any indication of becoming tumors or completely undifferentiated after OSK induction. A completely undifferentiated cell has no identity and is unable to perform specific tasks needed by mature cells. Avoiding such negative responses is critical to ensure the safety of potential medical uses of OSK.
One major age-related disease that affects RGCs is glaucoma, where patients experience significant vision loss and blindness. RGCs in the optic nerve of glaucoma patients gradually deteriorate, a characteristic of the disease the research team looked to reverse. RGCs of mice with glaucoma were treated by inducing OSK for one month. Under the microscope, treated RGCs of mice with glaucoma had the same axon density as mice without glaucoma. Furthermore, the treated glaucoma mice regained half of their visual acuity. With disease and injury-related methylation shown to be reversible, the researchers tested one final condition: natural aging. As with humans, the retinal ganglion cells of mice gradually accumulate points of methylation that worsen their vision. Aging mice with OSK-treated RGCs demonstrated restoration of visual acuity in mice up to twelve months old.
Altogether, this study introduces a potential avenue to treat age-related diseases in humans. Even though the researchers only investigated glaucoma, the results have promise to translate into a range of age-related disorders. Further research into its properties and functions holds a promising future for age-reversing therapies.
One major age-related disease that affects RGCs is glaucoma, where patients experience significant vision loss and blindness. RGCs in the optic nerve of glaucoma patients gradually deteriorate, a characteristic of the disease the research team looked to reverse. RGCs of mice with glaucoma were treated by inducing OSK for one month. Under the microscope, treated RGCs of mice with glaucoma had the same axon density as mice without glaucoma. Furthermore, the treated glaucoma mice regained half of their visual acuity. With disease and injury-related methylation shown to be reversible, the researchers tested one final condition: natural aging. As with humans, the retinal ganglion cells of mice gradually accumulate points of methylation that worsen their vision. Aging mice with OSK-treated RGCs demonstrated restoration of visual acuity in mice up to twelve months old.
Altogether, this study introduces a potential avenue to treat age-related diseases in humans. Even though the researchers only investigated glaucoma, the results have promise to translate into a range of age-related disorders. Further research into its properties and functions holds a promising future for age-reversing therapies.
RELATED ARTICLES
|
Vertical Divider
|
Vertical Divider
|
Vertical Divider
|