Personalized Nanorobots in Targeted Drug Delivery
Conventional cancer treatments like chemotherapy are non-specific and inaccurate, resulting in side effects from damaged healthy cells. However, a new approach called theranostics, which combines therapy and diagnostics, can potentially revolutionize personalized cancer medicine. It utilizes nanotechnology to more accurately target tumor cells for diagnosis, monitoring, and treatment without damaging other cells. In regards to drug delivery, “biohybrid microswimmers,” or motile microbes engineered with cargo carriers, are excellent candidates because of their ability to respond to environmental signals and to navigate complex, hard-to-reach places.
In one study, researchers created a biohybrid microswimmer consisting of E. coli conjugated with nanoerythrosomes, which are tiny red blood cells emptied of their contents to fulfill its function as cargo carriers. Streptavidin-biotin linkages, one of the strongest non-covalent bonds in nature, was used to attach the carriers to the bacteria. This was done by modifying E.coli and the nanoerythrosomes to bind to separate biotin molecules, more commonly known as Vitamin B7. These pairs were then be bound to a single streptavidin molecule, a protein that has high affinity for biotin.
In one study, researchers created a biohybrid microswimmer consisting of E. coli conjugated with nanoerythrosomes, which are tiny red blood cells emptied of their contents to fulfill its function as cargo carriers. Streptavidin-biotin linkages, one of the strongest non-covalent bonds in nature, was used to attach the carriers to the bacteria. This was done by modifying E.coli and the nanoerythrosomes to bind to separate biotin molecules, more commonly known as Vitamin B7. These pairs were then be bound to a single streptavidin molecule, a protein that has high affinity for biotin.
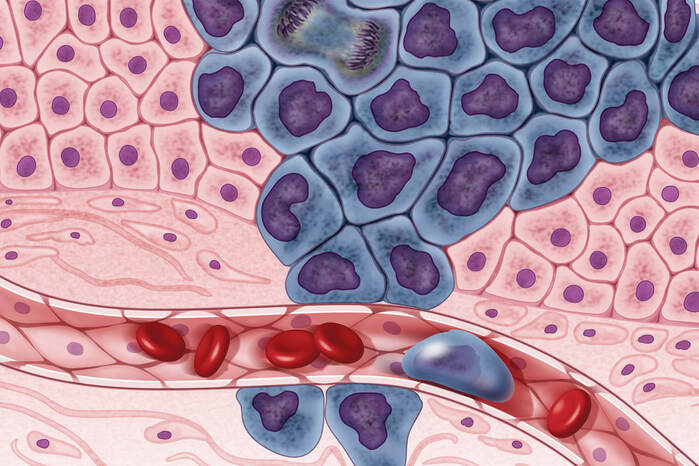
Image Source: "Cancer cells illustration" by NIH Image Gallery is licensed under Public Domain Mark 1.0
To create these nanoerythrosomes, the researchers separated mouse blood into three layers according to size and density. Their goal was to isolate red blood cells (RBCs) from white blood cells and other debris via centrifugation. Next, the RBCs were treated with a procedure that temporarily created 100 nanometer (nm) pores in the cell membrane so that the inner contents would be emptied—making space for its cargo carrying function. Lastly, these RBCs were further filtered to only acquire nanovesicles ranging from 100-1000 nm.
After each processing step, the researchers also verified that the RBCs still had two essential membrane proteins: TER118 and CD47. These proteins are both significant since TER119 binds to biotin, which is one of the key players in the linkage between the carriers and E. coli as mentioned above. Meanwhile, CD47 is essential to preventing macrophage phagocytosis. In other words, the RBCs are protected from destruction by the immune system. This enables it to stay in circulation for a longer time, optimizing it for potential long-term medical applications.
Finally, these nanoerythrosomes were conjugated with biotin and added to streptavidin-modified E. coli, forming the strong streptavidin-biotin linkage.The now attached nanoerythrosomes could be seen as pockets on its carrier, the E. coli, which together formed the “biohybrid microswimmer.” Next, the researcher assessed the motility by injecting these microswimmers into microchannels and visualizing through fluorescence microscopy—its speed was much greater than previous integrated E.coli hybrid designs. One explanatory factor could be due to the smaller size and density of their carrier design; thus, fluids cannot impede their movement as much.This is especially advantageous in more confined, complicated biological environments.
Altogether, this microswimmer design capitalizes on the synthetic and inherent biological properties of each of its components. E.coli, for instance, was chosen because of its superior ability compared to other bacterial species to survive in the body for a long time. Meanwhile, the nanoerythrosomes were derived directly from patient-specific cells, so their membrane proteins could help protect the biohybrid robots from attack by the individual’s own immune system. Furthermore, the small size of nanoerythrosomes enhanced their speed and ability to navigate complex areas. While this technology must still overcome other technical challenges, like how these nanorobots will carry and release their cargo to targeted cells, it holds tremendous promise for future precision medicine applications.
After each processing step, the researchers also verified that the RBCs still had two essential membrane proteins: TER118 and CD47. These proteins are both significant since TER119 binds to biotin, which is one of the key players in the linkage between the carriers and E. coli as mentioned above. Meanwhile, CD47 is essential to preventing macrophage phagocytosis. In other words, the RBCs are protected from destruction by the immune system. This enables it to stay in circulation for a longer time, optimizing it for potential long-term medical applications.
Finally, these nanoerythrosomes were conjugated with biotin and added to streptavidin-modified E. coli, forming the strong streptavidin-biotin linkage.The now attached nanoerythrosomes could be seen as pockets on its carrier, the E. coli, which together formed the “biohybrid microswimmer.” Next, the researcher assessed the motility by injecting these microswimmers into microchannels and visualizing through fluorescence microscopy—its speed was much greater than previous integrated E.coli hybrid designs. One explanatory factor could be due to the smaller size and density of their carrier design; thus, fluids cannot impede their movement as much.This is especially advantageous in more confined, complicated biological environments.
Altogether, this microswimmer design capitalizes on the synthetic and inherent biological properties of each of its components. E.coli, for instance, was chosen because of its superior ability compared to other bacterial species to survive in the body for a long time. Meanwhile, the nanoerythrosomes were derived directly from patient-specific cells, so their membrane proteins could help protect the biohybrid robots from attack by the individual’s own immune system. Furthermore, the small size of nanoerythrosomes enhanced their speed and ability to navigate complex areas. While this technology must still overcome other technical challenges, like how these nanorobots will carry and release their cargo to targeted cells, it holds tremendous promise for future precision medicine applications.
Featured Image Source: qimono
RELATED ARTICLES
|
Vertical Divider
|
Vertical Divider
|
Vertical Divider
|