Monkeypox: Understanding the Disease and Tecovirimat as a Treatment
Monkeypox is a new viral disease facing the nation, and there are a lot of public misconceptions surrounding the disease. The virus is very similar to smallpox, and symptoms include rashes, fever, aches, and respiratory symptoms like coughing or a sore throat. Despite the fact that this disease is endemic, or native, in some countries, the recent outbreaks in Western nations have been closely associated with and generated stigma against men in the LGBTQ+ community, similar to the HIV/AIDS endemic in the 1980’s. Since the disease is primarily spread between biological men having sex with other biological men, this has been weaponized to stoke fears around the gay community. After the AIDS epidemic, scientists and policy makers worked together to find effective solutions and ensure that novel medications can be approved in emergency situations like this. As such, new treatments are being developed to keep everyone safe from the disease. Researchers have had promising results with the drug Tecovirimat as a possible treatment for the disease as well as the newly developed vaccine. However, the drug still has limitations as it has not yet been tested on humans.
Tecovirimat was previously only approved to treat smallpox, but may soon be approved to treat Monkeypox in humans. The drug works by blocking a particular viral protein that enables the virus to penetrate cells in the body. However, the drug was authorized to treat smallpox under the Food and Drug Administration’s (FDA) Animal Rule. This rule states that the drug hasn’t been tested on humans because it would be unethical to expose them to a fatal virus, but it has been tested on animal models that are similar enough to mimic human immune responses. Since smallpox and monkeypox trigger a similar immune response in the human body, scientists believed it would be valuable to evaluate its efficacy against monkeypox.
Tecovirimat was previously only approved to treat smallpox, but may soon be approved to treat Monkeypox in humans. The drug works by blocking a particular viral protein that enables the virus to penetrate cells in the body. However, the drug was authorized to treat smallpox under the Food and Drug Administration’s (FDA) Animal Rule. This rule states that the drug hasn’t been tested on humans because it would be unethical to expose them to a fatal virus, but it has been tested on animal models that are similar enough to mimic human immune responses. Since smallpox and monkeypox trigger a similar immune response in the human body, scientists believed it would be valuable to evaluate its efficacy against monkeypox.
But this could soon be turned around due to promising new research from the University of California Los Angeles (UCLA). Dr. Raphael Landovitz, an infectious disease expert, is currently studying the effects of Tecovirimat as a treatment for humans suffering from monkeypox. This interventional study is in phase 3 of its clinical trial, which means it is nearing completion and the results are expected to be written and published soon. The study is a random, double-blind placebo experiment involving male volunteers with confirmed monkeypox infections. After the results are published, there should be enough evidence to prove the efficacy of Tecovirimat as a viable treatment for monkeypox and soon approved for the general population by the FDA. Hopefully, we can expect news on whether or not Tecovirimat is a suitable treatment for monkeypox, and its widespread use can calm the disease outbreak.
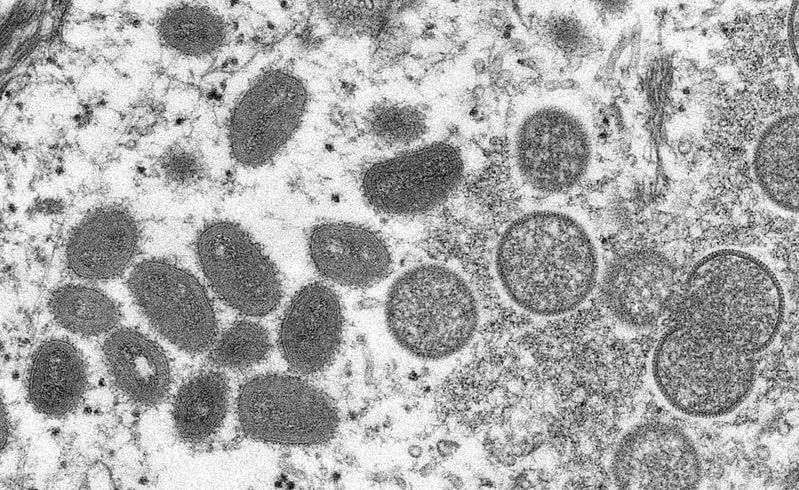
Featured Image Source: "Monkeypox Virion - CDC" by Cynthia Goldsmith
RELATED ARTICLES
|
Vertical Divider
|
Vertical Divider
|
Vertical Divider
|