Mitochondrial Calcium Transfer: A Potential Target for Cancer Therapy?
On a global scale, one in six people die from cancer, which remains the second leading cause of death in the world despite significant advancements in the development of cancer therapeutics. Cancer cells can develop in any part of the body, and these cells are generally distinguished by rapid proliferation. This uncontrolled growth often enables cancer cells to spread to other parts of the body as well as the breakdown of non-cancerous cells, which often leads to death.
The development of cancerous cells is generally thought to be driven by genetic mutations. However, there is growing evidence that cancer can also be characterized by important changes in metabolism that differentiate cancer cells from healthy cells. As described by the Warburg Effect, the processes that cancer cells use to generate energy often differ from the normal metabolic process commonly used by healthy cells for energy production.
As such, researchers seek to better understand the metabolic processes that are specifically used for energy production in cancer cells. Disrupting such processes could potentially result in cancer cell death, while minimally affecting healthy cells given the differences in their metabolic processes.
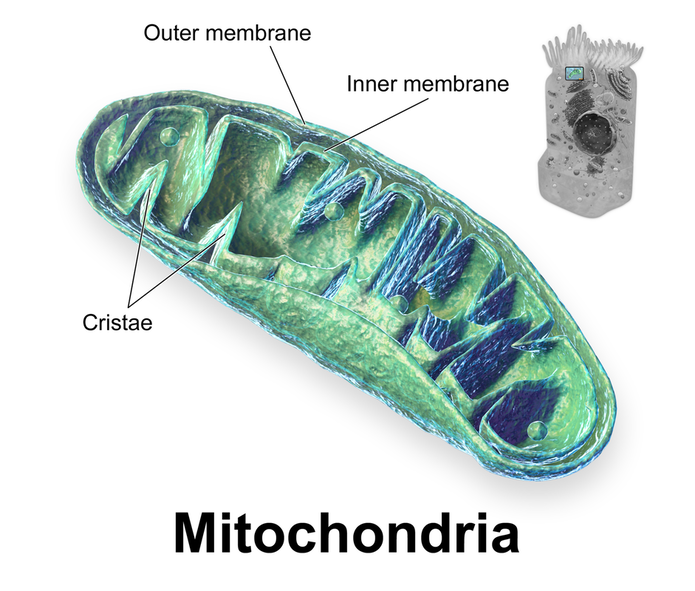
One metabolic process that specifically supports the growth and survival of many cancer cells is reductive carboxylation. To identify a potential target that could be used to block this process, a team of researchers decided to investigate calcium transfer from the endoplasmic reticulum (ER), the transport system of the cell, to the mitochondria where energy is produced.
The development of cancerous cells is generally thought to be driven by genetic mutations. However, there is growing evidence that cancer can also be characterized by important changes in metabolism that differentiate cancer cells from healthy cells. As described by the Warburg Effect, the processes that cancer cells use to generate energy often differ from the normal metabolic process commonly used by healthy cells for energy production.
As such, researchers seek to better understand the metabolic processes that are specifically used for energy production in cancer cells. Disrupting such processes could potentially result in cancer cell death, while minimally affecting healthy cells given the differences in their metabolic processes.
One metabolic process that specifically supports the growth and survival of many cancer cells is reductive carboxylation. To identify a potential target that could be used to block this process, a team of researchers decided to investigate calcium transfer from the endoplasmic reticulum (ER), the transport system of the cell, to the mitochondria where energy is produced.
Calcium transfer from the ER to the mitochondria is an important process that helps maintain energy production in both healthy and cancer cells, despite the different metabolic processes used for energy production in both cell types. Since this process of calcium transfer is also present in healthy cells, the researchers needed to experimentally determine whether blocking the entry of calcium from the ER into the mitochondria could selectively lead to cancer cell death without also killing healthy cells with normal energy metabolism.
To stop calcium transfer from the ER to the mitochondria, the researchers used an inhibitor specific to a calcium channel which would prevent the release of calcium from the ER. The researchers found that in cells where calcium transfer from the ER to the mitochondria is inhibited, prosurvival mechanisms, whereby the cell removes its dysfunctional or unnecessary components, are induced. While these prosurvival mechanisms were sufficient to actually promote the survival of healthy cells with normal energy metabolism, the inhibition of calcium transfer from the ER to the mitochondria ultimately increased the likelihood of death in cells that relied on the same reductive carboxylation process used by many cancer cells for energy production.
In other words, these experimental results demonstrate that calcium transfer from the ER to the mitochondria is required for the survival of cancer cells that rely on reductive carboxylation for energy production, but it is not necessarily required for the survival of healthy cells with normal energy metabolism. This suggests that inhibiting calcium transfer from the ER to the mitochondria could potentially be used as a therapeutic strategy to specifically target cancer cells without harming healthy cells with normal energy metabolism.
While further research is necessary to determine how these findings can be applied for the benefit of human health, this research illustrates how a better understanding of cancer cell metabolism could greatly contribute to the development of novel cancer treatments and prevention efforts.
To stop calcium transfer from the ER to the mitochondria, the researchers used an inhibitor specific to a calcium channel which would prevent the release of calcium from the ER. The researchers found that in cells where calcium transfer from the ER to the mitochondria is inhibited, prosurvival mechanisms, whereby the cell removes its dysfunctional or unnecessary components, are induced. While these prosurvival mechanisms were sufficient to actually promote the survival of healthy cells with normal energy metabolism, the inhibition of calcium transfer from the ER to the mitochondria ultimately increased the likelihood of death in cells that relied on the same reductive carboxylation process used by many cancer cells for energy production.
In other words, these experimental results demonstrate that calcium transfer from the ER to the mitochondria is required for the survival of cancer cells that rely on reductive carboxylation for energy production, but it is not necessarily required for the survival of healthy cells with normal energy metabolism. This suggests that inhibiting calcium transfer from the ER to the mitochondria could potentially be used as a therapeutic strategy to specifically target cancer cells without harming healthy cells with normal energy metabolism.
While further research is necessary to determine how these findings can be applied for the benefit of human health, this research illustrates how a better understanding of cancer cell metabolism could greatly contribute to the development of novel cancer treatments and prevention efforts.
Featured Image Source: geralt
RELATED ARTICLES
|
Vertical Divider
|
Vertical Divider
|
Vertical Divider
|