Detecting SARS-CoV-2: Comparing Molecular and Antigen Testing
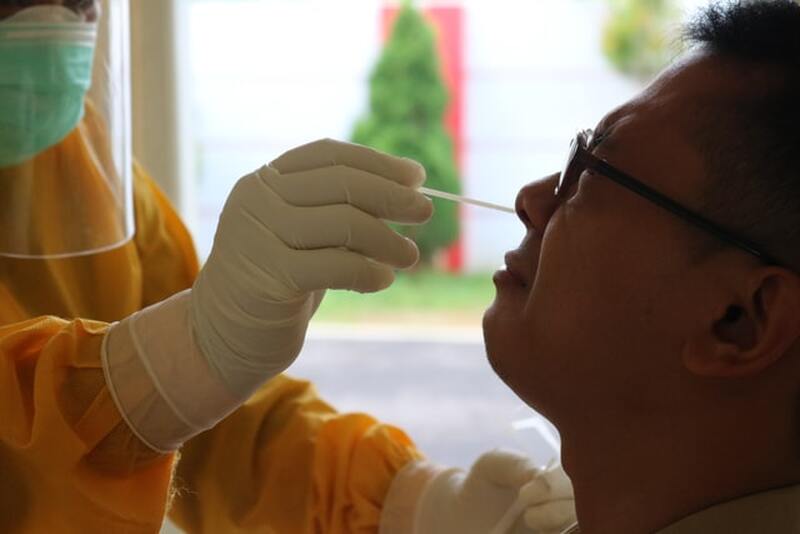
In order to contain the spread of COVID-19, it is critical to identify infected individuals as early as possible. This can only be done through diagnostic testing of the target population, which can show if someone is actively infected. Currently, there are two types of diagnostic tests used in the US to detect SARS-CoV-2, the virus that causes COVID-19. One type is the molecular test, which looks for SARS-CoV-2 genetic material in the body, and the other is the antigen test, which looks for the presence of viral proteins. According to a study published in the Journal of Clinical Virology, there are different benefits and potential risks associated with each test. Since reliable tests are vital to prevent the virus’s spread, this research is essential for establishing testing sites and identifying COVID-19 hotspots.
The most common type of molecular test is the Reverse Transcription Polymerase Chain Reaction (RT-PCR) test, which generally uses samples from the nose, throat, or nasopharyngeal region (the throat area behind the nose). Occasionally, saliva samples can be used. To analyze a swab using polymerase chain reactions (PCR), viral RNA is extracted from the sample, converted to DNA, then copied multiple times via PCR. Probes made with viral genetic material are then used to interact with the PCR-produced DNA samples. If the individual currently has viral genetic material in their body, then these probes will attach to the complementary DNA and light up the sample, indicating a positive test result. The sensitivity of molecular tests varies, but research suggests current PCR tests detect the virus with at least 95% sensitivity. The main disadvantage of molecular testing is that the process can be time-consuming. While certain RT-PCR tests can be analyzed at the testing site for same-day results, most must be sent to an outside laboratory for analysis and take up to several days to produce a result.
The most common type of molecular test is the Reverse Transcription Polymerase Chain Reaction (RT-PCR) test, which generally uses samples from the nose, throat, or nasopharyngeal region (the throat area behind the nose). Occasionally, saliva samples can be used. To analyze a swab using polymerase chain reactions (PCR), viral RNA is extracted from the sample, converted to DNA, then copied multiple times via PCR. Probes made with viral genetic material are then used to interact with the PCR-produced DNA samples. If the individual currently has viral genetic material in their body, then these probes will attach to the complementary DNA and light up the sample, indicating a positive test result. The sensitivity of molecular tests varies, but research suggests current PCR tests detect the virus with at least 95% sensitivity. The main disadvantage of molecular testing is that the process can be time-consuming. While certain RT-PCR tests can be analyzed at the testing site for same-day results, most must be sent to an outside laboratory for analysis and take up to several days to produce a result.
Image Source: Martin Lopez
In contrast to molecular RT-PCR tests, rapid antigen tests consistently yield results in under an hour. Samples for these can be taken from nasal and nasopharyngeal regions. Instead of detecting viral genetic material like PCR tests, these tests search for antigens, which are viral proteins that attach to and infect healthy cells in the body. Because antigen tests only look for surface proteins, they do not require lengthy lab procedures for analysis. Instead, administrators extract the specimen from the swab and insert it in a testing device, which produces a positive result in 15-30 minutes if SARS-CoV-2 antigens are detected. When used on individuals confirmed positive for COVID-19 through RT-PCR, research suggests antigen testing has a sensitivity of 73.3%, meaning it detects the virus roughly three quarters of the time that PCR does. Rapid antigen testing does have a higher sensitivity when used within an individual’s first week of symptoms, which is when an infected individual is most contagious. In these instances, the sensitivity of antigen tests rises to 86.5%, making them more competitive with PCR testing.
While PCR tests remain the gold standard for identifying SARS-CoV-2, antigen testing can still be worthwhile in certain settings. Rapid antigen testing can be useful for screening large groups of high-risk individuals in a short period of time, especially if these individuals have been exposed to the virus within the past week. Even if testing methods cannot detect the virus with 100% accuracy, frequent testing is crucial to contain the virus. Although the distribution of SARS-CoV-2 vaccines is predicted to occur in the coming months, widespread testing and quarantining upon a positive result can prevent high-risk individuals from contracting the disease at all.
While PCR tests remain the gold standard for identifying SARS-CoV-2, antigen testing can still be worthwhile in certain settings. Rapid antigen testing can be useful for screening large groups of high-risk individuals in a short period of time, especially if these individuals have been exposed to the virus within the past week. Even if testing methods cannot detect the virus with 100% accuracy, frequent testing is crucial to contain the virus. Although the distribution of SARS-CoV-2 vaccines is predicted to occur in the coming months, widespread testing and quarantining upon a positive result can prevent high-risk individuals from contracting the disease at all.
Featured Image Source: Mufid Majnun
RELATED ARTICLES
|
Vertical Divider
|
Vertical Divider
|
Vertical Divider
|