Can CRISPR-Cas9 Genome Editing Improve Cancer Immunotherapy?
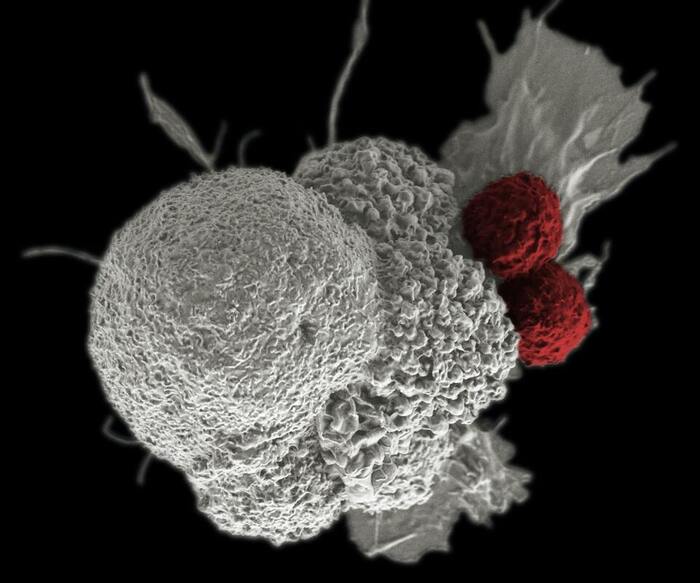
The human body’s immune system has natural antitumor activity that can be manipulated for higher efficiency and specificity. In fact, this is the idea behind cancer immunotherapy. There are immune cells called T cells that circulate in the bloodstream, detect toxins and foreign substances, and signal the immune system for an attack. Adoptive T cell therapy, a type of immunotherapy, uses T cells with genetically engineered, transgenic T cell receptors (TCRs). TCRs, located on the cell surface, contain two protein structures, α and β chains, which are important for recognizing antigens, or proteins from foreign or toxic substances. Previous research has shown some progress in manufacturing T cells with transgenic TCRs specifically targeting the tumor antigen NY-ESO1.
However, there are limitations to this approach. The transgenic α and β chains often mispaired or competed with the original chains produced by the T cells, which can then lead to T cell dysfunction. Moreover, additional research shows that the programmed cell death protein 1 (PD1), a normal cellular protein, hinders the T cell antitumor response and presents another limitation to adoptive T cell therapy. To solve these problems, researchers utilized CRISPR-cas9 genome editing to disrupt the expression of the original α and β chains and PD1 in the transgenic T cells. Their research, explored CRISPR editing as a means to improve the safety and efficacy of adoptive T cell therapy using NY-ESO-1 specific TCRs.
However, there are limitations to this approach. The transgenic α and β chains often mispaired or competed with the original chains produced by the T cells, which can then lead to T cell dysfunction. Moreover, additional research shows that the programmed cell death protein 1 (PD1), a normal cellular protein, hinders the T cell antitumor response and presents another limitation to adoptive T cell therapy. To solve these problems, researchers utilized CRISPR-cas9 genome editing to disrupt the expression of the original α and β chains and PD1 in the transgenic T cells. Their research, explored CRISPR editing as a means to improve the safety and efficacy of adoptive T cell therapy using NY-ESO-1 specific TCRs.
This study was a pilot human phase 1 clinical trial of three patients. T cells taken from the patients were genetically modified and infused back into the patients. DNA breaks were induced at the three genes of interest: TRAC, TRBC, and PDCD1. This then disrupted protein expression of α chain, β chain, and PD1 in the T cells, respectively. When these genetically engineered T cells were cultured with tumor cells expressing NY-ESO-1 (the tumor antigen), the engineered T cells showed potent activity damaging the tumor cells. After infusion, the engineered T cells persisted well in the three patients as seen in blood cell counts that were stable for a few months. CRISPR editing was also highly specific, rarely disrupting other genes in the cells.
The three patients showed no signs of adverse side effects or immune responses. Two patients experienced halted tumor growth, while one patient experienced abdominal tumor regression but growth of other lesions. Examination of tumor cells showed that the engineered T cells were trafficked to tumor sites in all three patients. Of the three patients, the blood cells taken from the previously mentioned patient exhibited the most prominent antitumor activity when cultured with NY-ESO-1, consistent with the observation of tumor regression.
This study supports the compatibility of CRISPR-cas9 genome editing with adoptive T cell therapy. However, limitations to the experimental approach make the results mostly inconclusive and call for more extensive research in the future. Scientists need to confirm not only the safety of this treatment but also whether the disruption of PD1 had a positive effect. Additionally, the high precision of CRISPR editing does not take away the occasional disruption of other proteins, and the DNA breaks may also cause chromosomal rearrangements that may be dangerous. Although adoptive T cell therapy has shown some ability to treat cancer, it is still limited in scope because all three patients in the study faced disease progression, of which one passed away. Fortunately, new reagents and protocols are under development that may greatly improve the efficiency of this approach.
The three patients showed no signs of adverse side effects or immune responses. Two patients experienced halted tumor growth, while one patient experienced abdominal tumor regression but growth of other lesions. Examination of tumor cells showed that the engineered T cells were trafficked to tumor sites in all three patients. Of the three patients, the blood cells taken from the previously mentioned patient exhibited the most prominent antitumor activity when cultured with NY-ESO-1, consistent with the observation of tumor regression.
This study supports the compatibility of CRISPR-cas9 genome editing with adoptive T cell therapy. However, limitations to the experimental approach make the results mostly inconclusive and call for more extensive research in the future. Scientists need to confirm not only the safety of this treatment but also whether the disruption of PD1 had a positive effect. Additionally, the high precision of CRISPR editing does not take away the occasional disruption of other proteins, and the DNA breaks may also cause chromosomal rearrangements that may be dangerous. Although adoptive T cell therapy has shown some ability to treat cancer, it is still limited in scope because all three patients in the study faced disease progression, of which one passed away. Fortunately, new reagents and protocols are under development that may greatly improve the efficiency of this approach.
Featured Image Source: National Cancer Institute
RELATED ARTICLES
|
Vertical Divider
|
Vertical Divider
|
Vertical Divider
|