A New Potential Drug Target to Prevent Osteoporosis
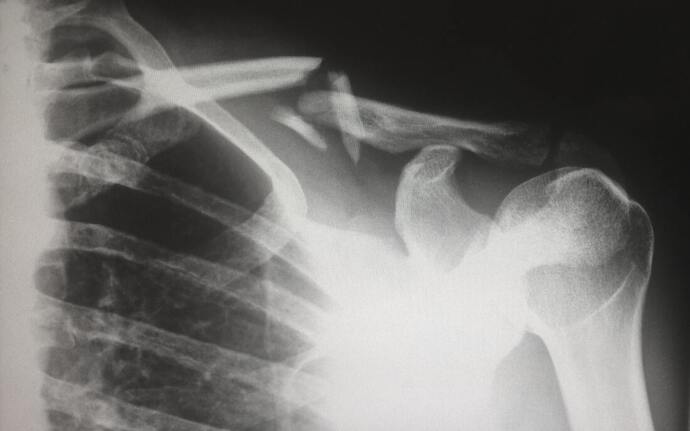
In the United States, osteoporosis, a severe weakening of bones, is one of the most common conditions affecting the aging population. To understand osteoporosis, it is helpful to break down the word: “osteo” means bone, “poro” means porous or passage, and “osis” means a state or disease. A diagnosis of osteoporosis requires the person to have a bone density at least 2.5 standard deviations lower than the healthy young adult average. According to the CDC, osteoporosis affects 5.1% of men and 24.5% of women 65 years of age or older. Women are at increased risk of developing osteoporosis due to having smaller bones than men. The risk increases after hormonal shifts that occur during menopause. However, estimates show that one in four men over 50 will break a bone due to osteoporosis, which can be difficult to diagnose because the first clear sign of osteoporosis is often bone fracture.
Many people think of bones as permanent and unchanging. In fact, bones are constantly being remodeled. Old bone is regularly broken down by cells called osteoclasts so that new bone can be replaced by cells called osteoblasts. When osteoclasts degrade bone faster than osteoblasts make new bone, bone density can decrease to dangerously low levels, resulting in osteoporosis. The underlying cause of this imbalance of osteoclast and osteoblast function is varied and not well understood. Therefore, research in these two bone cells gives important insights into how medicine may be able to prevent osteoporosis.
Many people think of bones as permanent and unchanging. In fact, bones are constantly being remodeled. Old bone is regularly broken down by cells called osteoclasts so that new bone can be replaced by cells called osteoblasts. When osteoclasts degrade bone faster than osteoblasts make new bone, bone density can decrease to dangerously low levels, resulting in osteoporosis. The underlying cause of this imbalance of osteoclast and osteoblast function is varied and not well understood. Therefore, research in these two bone cells gives important insights into how medicine may be able to prevent osteoporosis.
Image Source: Harlie Raethel
Considering this, the Qin Lab at University of Pennsylvania’s Perelman School of Medicine recently made a key breakthrough about a potential explanation for the progression of osteoporosis. Previous experiments conducted in the Qin Lab identified a new type of cell called marrow adipogenic lineage precursors (MALPs). The Qin Lab’s follow-up study showed that MALPs release molecules that trigger the production of osteoclasts, which was unexpected to the researchers.
The lab then turned to rat models to manipulate MALPs and the molecules they release to further understand their role in bone remodeling. First, they created a rat with MALPs that do not release one of the key molecules, RANKL, for osteoclast formation. In these rats, fewer osteoclasts were present. Subsequently, because osteoblasts became more prevalent than osteoclasts, there was an increase in overall bone mass. Next, they tested the effect of MALPs on rat models of bacteria-induced and postmenopausal osteoporosis. With the bacteria-induced osteoporosis model, eliminating RANKL release from MALPs prevented the bone destruction that occurred in control rats that were not treated. Further analysis showed 34 times more osteoclasts in control rats, indicating the powerful effect of RANKL release from MALPs on the breakdown of bone. In the postmenopausal model, elimination of RANKL release from MALPs also decreased osteoclast number and surface area, though to a lesser extent.
This new research reveals a new understanding of osteoporosis and points to targets for future drug therapies. Currently, drugs that slow the osteoclasts’ function are the most common therapies for osteoporosis. However, these drugs do not assure complete prevention of bone breakdown and are associated with several unwanted effects, such as osteonecrosis, the death of bone cells. Therefore, targeting MALPs has the potential to prevent bone destruction while also avoiding the negative side effects of current medications. For this to happen, more research needs to be done on the role of MALPs in other areas of bone regulation and how to disable RANKL release from MALPs. If this can be done, the balance between bone breakdown and bone formation could be restored, potentially helping millions of people from the debilitating effects of osteoporosis.
The lab then turned to rat models to manipulate MALPs and the molecules they release to further understand their role in bone remodeling. First, they created a rat with MALPs that do not release one of the key molecules, RANKL, for osteoclast formation. In these rats, fewer osteoclasts were present. Subsequently, because osteoblasts became more prevalent than osteoclasts, there was an increase in overall bone mass. Next, they tested the effect of MALPs on rat models of bacteria-induced and postmenopausal osteoporosis. With the bacteria-induced osteoporosis model, eliminating RANKL release from MALPs prevented the bone destruction that occurred in control rats that were not treated. Further analysis showed 34 times more osteoclasts in control rats, indicating the powerful effect of RANKL release from MALPs on the breakdown of bone. In the postmenopausal model, elimination of RANKL release from MALPs also decreased osteoclast number and surface area, though to a lesser extent.
This new research reveals a new understanding of osteoporosis and points to targets for future drug therapies. Currently, drugs that slow the osteoclasts’ function are the most common therapies for osteoporosis. However, these drugs do not assure complete prevention of bone breakdown and are associated with several unwanted effects, such as osteonecrosis, the death of bone cells. Therefore, targeting MALPs has the potential to prevent bone destruction while also avoiding the negative side effects of current medications. For this to happen, more research needs to be done on the role of MALPs in other areas of bone regulation and how to disable RANKL release from MALPs. If this can be done, the balance between bone breakdown and bone formation could be restored, potentially helping millions of people from the debilitating effects of osteoporosis.
Featured Image Source: StockSnap / 27557 images
RELATED ARTICLES
|
Vertical Divider
|
Vertical Divider
|
Vertical Divider
|